What is a chelating agent milady – Embarking on a scientific expedition into the enigmatic realm of chelating agents, this discourse unravels the intricate nature of these molecular grabbers. Chelating agents, with their remarkable ability to sequester metal ions, play a pivotal role in diverse fields, from industry to medicine and environmental remediation.
Join us as we delve into the captivating world of chelating agents, deciphering their characteristics, applications, and environmental implications.
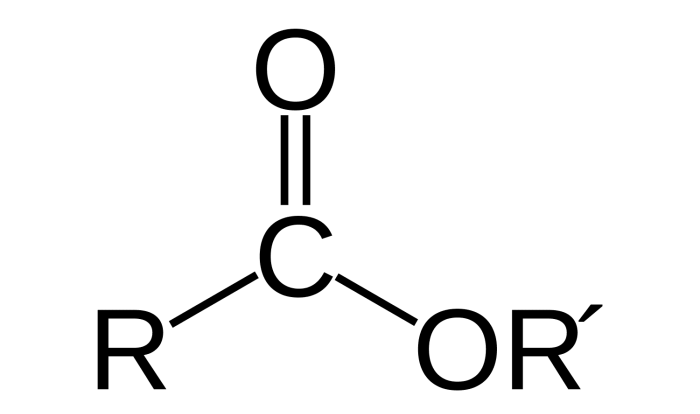
Chelating agents, also known as chelators, are organic molecules that possess a unique structural feature—multiple coordinating groups. These coordinating groups, often comprising oxygen, nitrogen, or sulfur atoms, form strong bonds with metal ions, effectively encasing them within their molecular embrace.
This remarkable property endows chelating agents with the ability to control the reactivity and fate of metal ions in various chemical systems.
1. Introduction
Chelating agents are chemical compounds that have the ability to form stable, water-soluble complexes with metal ions. This property makes them useful in a wide range of industrial and medical applications.
Examples of chelating agents include ethylenediaminetetraacetic acid (EDTA), nitrilotriacetic acid (NTA), and diethylenetriaminepentaacetic acid (DTPA).
2. Characteristics of Chelating Agents
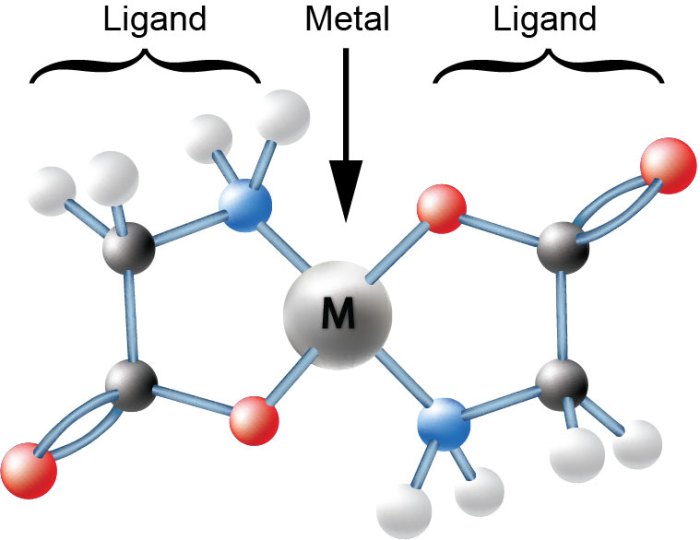
2.1 Structure and Properties
Chelating agents typically have multiple functional groups, such as amino, carboxyl, and hydroxyl groups, that can coordinate with metal ions. These functional groups form coordinate bonds with the metal ion, resulting in the formation of a stable complex.
The stability of the chelate complex depends on the following factors:
- The number and type of functional groups present in the chelating agent
- The size and charge of the metal ion
- The pH of the solution
2.2 Factors Affecting the Effectiveness of Chelating Agents
The effectiveness of a chelating agent is determined by its ability to form a stable complex with the metal ion of interest. The following factors can affect the effectiveness of a chelating agent:
- The stability constant of the chelate complex
- The concentration of the chelating agent
- The presence of other metal ions in the solution
- The pH of the solution
3. Applications of Chelating Agents: What Is A Chelating Agent Milady
3.1 Industrial Applications
Chelating agents are used in a variety of industrial applications, including:
- Water treatment: Chelating agents are used to remove metal ions from water, such as calcium and magnesium ions, which can cause scaling in pipes and boilers.
- Textile industry: Chelating agents are used to remove metal ions from textiles, such as iron and copper ions, which can cause discoloration and damage to the fabric.
- Paper industry: Chelating agents are used to remove metal ions from paper pulp, which can cause discoloration and weaken the paper.
3.2 Medical Applications, What is a chelating agent milady
Chelating agents are used in a variety of medical applications, including:
- Treatment of metal poisoning: Chelating agents can be used to remove toxic metals from the body, such as lead, mercury, and arsenic.
- Treatment of kidney stones: Chelating agents can be used to dissolve kidney stones, which are composed of calcium oxalate or calcium phosphate.
- Treatment of iron overload: Chelating agents can be used to remove excess iron from the body, which can be caused by certain diseases, such as thalassemia and sickle cell anemia.
4. Environmental Implications
Chelating agents can have negative environmental impacts if they are not used properly. These impacts include:
- Mobility of metal ions: Chelating agents can increase the mobility of metal ions in the environment, which can lead to their accumulation in plants and animals.
- Toxicity to aquatic organisms: Chelating agents can be toxic to aquatic organisms, such as fish and shellfish.
4.1 Solutions for Mitigating the Environmental Impact of Chelating Agents
The environmental impact of chelating agents can be mitigated by:
- Using chelating agents in a controlled manner
- Recycling chelating agents
- Developing biodegradable chelating agents
Question Bank
What are the key characteristics of chelating agents?
Chelating agents are characterized by their ability to form multiple coordinate bonds with metal ions, creating stable complexes. They typically have a high affinity for metal ions and can effectively sequester them from solution.
What are some common examples of chelating agents?
EDTA (ethylenediaminetetraacetic acid), NTA (nitrilotriacetic acid), and citric acid are widely used chelating agents. These compounds have been employed in various industrial, medical, and environmental applications.
How do chelating agents contribute to industrial processes?
Chelating agents play a crucial role in industrial water treatment, metal extraction, and textile dyeing. They prevent the formation of scale and corrosion in water systems, facilitate the selective extraction of metals from ores, and enhance the colorfastness of dyes.
What are the medical applications of chelating agents?
Chelating agents are used in medicine to treat metal poisoning, remove excess iron from the body, and enhance the delivery of drugs to specific tissues. They have also shown promise in the development of new cancer therapies.
What are the potential environmental implications of chelating agents?
While chelating agents are essential for various applications, their release into the environment can pose ecological concerns. They can mobilize heavy metals from soils and sediments, potentially leading to their accumulation in aquatic ecosystems.