The Grignard reaction synthesis of triphenylmethanol is a powerful technique in organic chemistry that enables the synthesis of complex organic molecules. This reaction involves the addition of a Grignard reagent to a carbonyl group, resulting in the formation of a new carbon-carbon bond.
In this article, we will delve into the detailed mechanism of the Grignard reaction, explore the specific steps involved in synthesizing triphenylmethanol using this method, and discuss the factors that affect the yield and selectivity of the reaction.
The Grignard reaction is a versatile tool that has found applications in various fields of chemistry, including organic synthesis, materials science, and pharmaceutical chemistry. By understanding the fundamental principles and optimization strategies of the Grignard reaction, chemists can effectively utilize this technique to synthesize a wide range of valuable organic compounds.
Grignard Reaction Mechanism
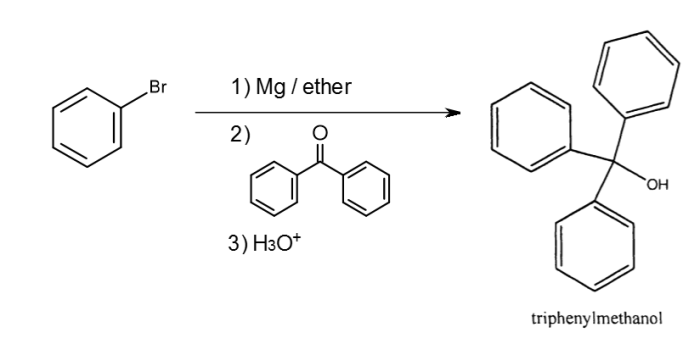
The Grignard reaction is a powerful tool for the synthesis of various organic compounds. It involves the addition of an organometallic compound, known as a Grignard reagent, to a carbonyl group. The Grignard reagent is typically formed by the reaction of an alkyl or aryl halide with magnesium metal in an ethereal solvent.
Formation of the Grignard Reagent
The formation of the Grignard reagent is a two-step process. In the first step, the alkyl or aryl halide undergoes oxidative addition to magnesium metal, resulting in the formation of a highly reactive organomagnesium halide intermediate. In the second step, this intermediate undergoes a transmetallation reaction with another molecule of the alkyl or aryl halide, leading to the formation of the Grignard reagent.
Nucleophilic Addition to the Carbonyl Group, Grignard reaction synthesis of triphenylmethanol
The Grignard reagent acts as a nucleophile and adds to the carbonyl group of an aldehyde or ketone. This addition occurs in a two-step process. In the first step, the nucleophilic Grignard reagent attacks the electrophilic carbon of the carbonyl group, forming a tetrahedral intermediate.
In the second step, this intermediate collapses, expelling the magnesium halide and forming a new carbon-carbon bond.
Synthesis of Triphenylmethanol: Grignard Reaction Synthesis Of Triphenylmethanol
Triphenylmethanol is a tertiary alcohol that can be synthesized using the Grignard reaction. The reaction involves the addition of phenylmagnesium bromide, the Grignard reagent, to benzaldehyde, an aldehyde.
The reaction is typically carried out in an ethereal solvent, such as diethyl ether or tetrahydrofuran, under anhydrous conditions. The reaction mixture is cooled to a low temperature, typically around -78 °C, to prevent side reactions.
The reaction proceeds via the nucleophilic addition of the Grignard reagent to the carbonyl group of benzaldehyde, followed by protonation of the resulting alkoxide intermediate to give triphenylmethanol.
Reaction Optimization
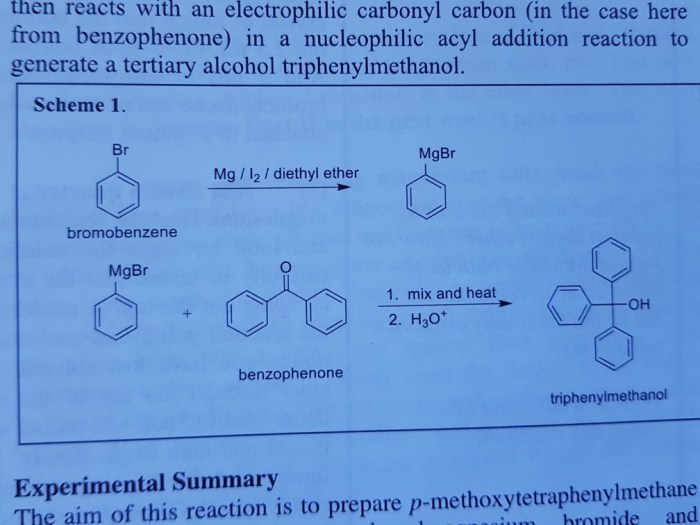
The yield and selectivity of the Grignard reaction can be optimized by controlling various reaction parameters, such as the choice of Grignard reagent, solvent, and reaction temperature.
The choice of Grignard reagent can affect the reactivity and selectivity of the reaction. For example, Grignard reagents derived from primary alkyl halides are typically more reactive than those derived from secondary or tertiary alkyl halides. Additionally, the use of bulky Grignard reagents can help to improve the selectivity of the reaction by reducing the likelihood of side reactions.
The solvent used in the reaction can also affect the yield and selectivity. Ethereal solvents, such as diethyl ether and tetrahydrofuran, are commonly used because they solvate the Grignard reagent and help to prevent side reactions.
The reaction temperature can also affect the yield and selectivity of the reaction. Lower reaction temperatures typically favor the formation of the desired product, while higher reaction temperatures can lead to side reactions.
Applications of Triphenylmethanol
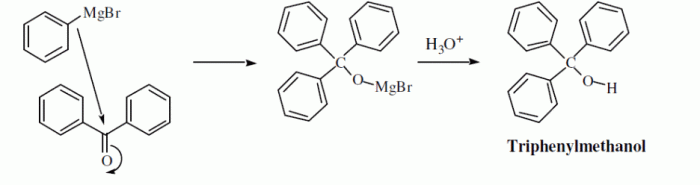
Triphenylmethanol is a versatile compound with a wide range of applications. It is used as an intermediate in the synthesis of various organic compounds, including dyes, pharmaceuticals, and fragrances.
Triphenylmethanol is also used as a starting material for the preparation of other compounds, such as triphenylmethyl chloride, which is a useful reagent in organic synthesis.
Common Queries
What is the mechanism of the Grignard reaction?
The Grignard reaction proceeds through a nucleophilic addition mechanism. The Grignard reagent, which is an organometallic compound, attacks the electrophilic carbonyl group, forming a new carbon-carbon bond.
What are the factors that affect the yield and selectivity of the Grignard reaction?
The yield and selectivity of the Grignard reaction can be affected by various factors, including the nature of the Grignard reagent, the solvent, the reaction temperature, and the presence of additives.
What are the applications of triphenylmethanol?
Triphenylmethanol is a versatile intermediate in organic synthesis. It can be used to prepare a variety of other compounds, including dyes, pharmaceuticals, and polymers.