Embark on an intriguing exploration of organic compounds, the building blocks of life and countless everyday products. Through the lens of a class of organic compounds crossword, we unravel the intricate chemical structures, diverse classifications, and remarkable properties that define these fascinating molecules.
Organic compounds, a vast and multifaceted group, captivate scientists and industry professionals alike. Their unique characteristics stem from their carbon-based skeletons, adorned with various functional groups that orchestrate their behavior and reactivity.
Definition of Organic Compounds: A Class Of Organic Compounds Crossword
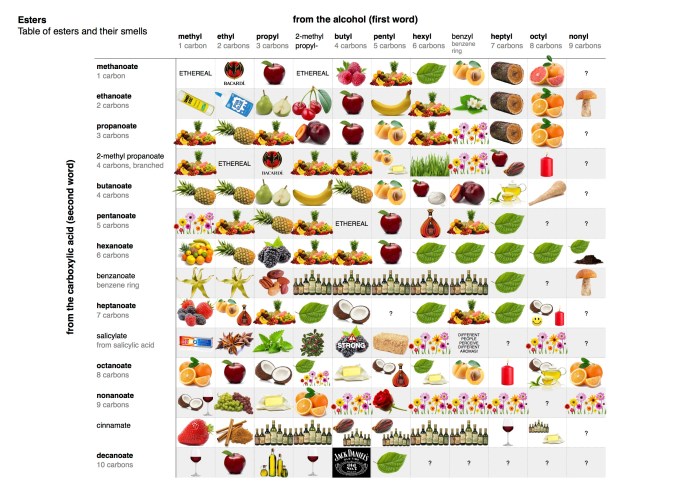
Organic compounds are a class of chemical compounds that contain carbon. They are the basis of all life on Earth and are found in a wide variety of natural and synthetic materials.
The chemical structure of organic compounds is based on a carbon backbone. Carbon atoms can form single, double, or triple bonds with each other and with other atoms, such as hydrogen, oxygen, nitrogen, and sulfur. This allows for a vast array of possible structures and properties.
Types of Organic Compounds, A class of organic compounds crossword
- Aliphatic compounds: These compounds contain carbon atoms arranged in a straight chain or branched chain.
- Aromatic compounds: These compounds contain carbon atoms arranged in a ring structure.
- Heterocyclic compounds: These compounds contain carbon atoms arranged in a ring structure that also includes one or more atoms of another element, such as nitrogen, oxygen, or sulfur.
Examples of organic compounds include methane, ethane, benzene, toluene, and glucose.
Classification of Organic Compounds
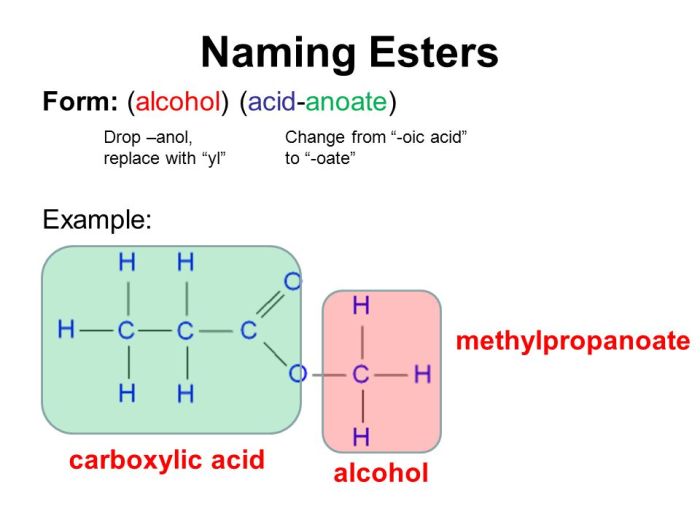
Organic compounds are classified into different groups based on their structure and properties. The main classes of organic compounds are:
- Alkanes: These compounds contain only carbon and hydrogen atoms and have a single bond between each carbon atom.
- Alkenes: These compounds contain carbon and hydrogen atoms and have one or more double bonds between carbon atoms.
- Alkynes: These compounds contain carbon and hydrogen atoms and have one or more triple bonds between carbon atoms.
- Alcohols: These compounds contain a hydroxyl group (-OH) attached to a carbon atom.
- Ethers: These compounds contain an oxygen atom bonded to two carbon atoms.
- Aldehydes: These compounds contain a carbonyl group (-C=O) attached to a carbon atom.
- Ketones: These compounds contain a carbonyl group (-C=O) attached to two carbon atoms.
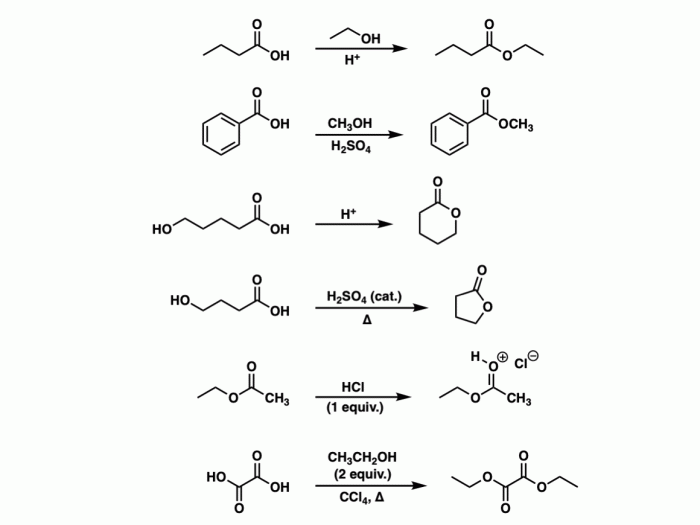
- Carboxylic acids: These compounds contain a carboxyl group (-COOH) attached to a carbon atom.
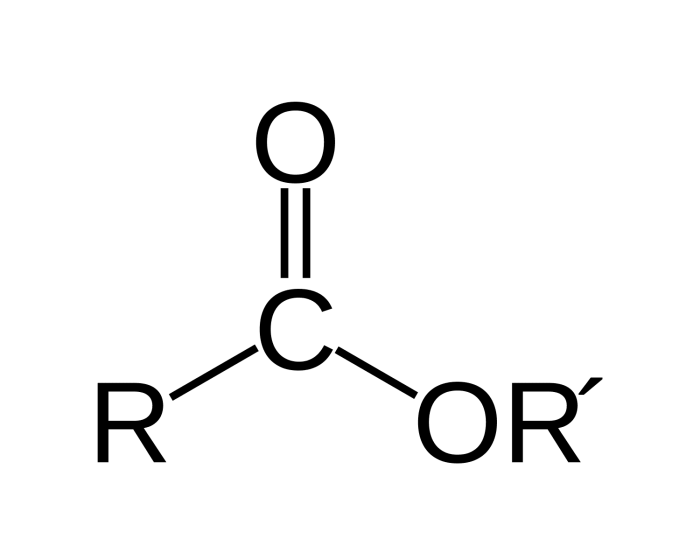
- Esters: These compounds contain a carboxyl group (-COOH) attached to an oxygen atom, which is in turn attached to a carbon atom.
- Amides: These compounds contain a nitrogen atom bonded to a carbonyl group (-C=O).
The following table summarizes the different classes of organic compounds:
| Class | Structure | Properties |
|---|---|---|
| Alkanes | CnH2n+2 | Saturated hydrocarbons |
| Alkenes | CnH2n | Unsaturated hydrocarbons |
| Alkynes | CnH2n-2 | Unsaturated hydrocarbons |
| Alcohols | ROH | Polar solvents |
| Ethers | ROR’ | Nonpolar solvents |
| Aldehydes | RCHO | Reactive carbonyl compounds |
| Ketones | RCOR’ | Reactive carbonyl compounds |
| Carboxylic acids | RCOOH | Weak acids |
| Esters | RCOOR’ | Polar solvents |
| Amides | RCONH2 | Polar compounds |
Commonly Asked Questions
What are the key characteristics of organic compounds?
Organic compounds are characterized by their carbon-based skeletons, covalently bonded to hydrogen, oxygen, nitrogen, and other elements. They exhibit a wide range of molecular structures and functional groups, giving rise to their diverse properties.
How are organic compounds classified?
Organic compounds are classified based on their functional groups, which determine their chemical reactivity and physical properties. Common classes include hydrocarbons, alcohols, aldehydes, ketones, and carboxylic acids.
What are some examples of organic compounds?
Organic compounds are ubiquitous in nature and industry. Examples include methane (natural gas), ethanol (alcohol), glucose (sugar), and polyethylene (plastic).